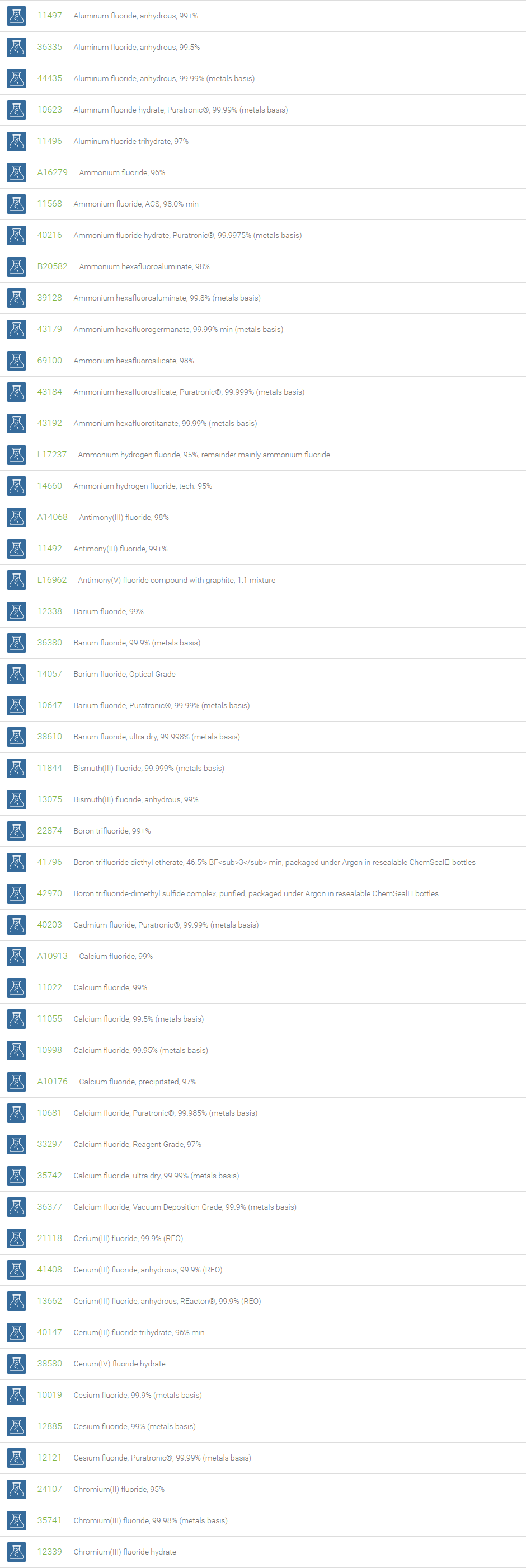
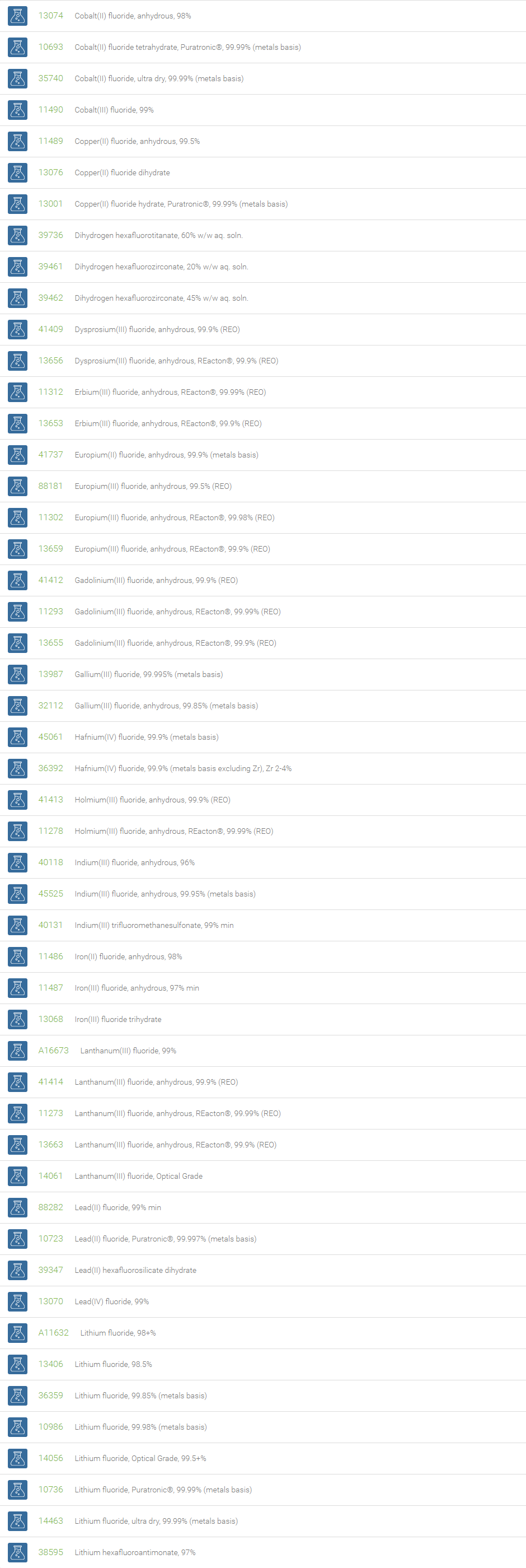
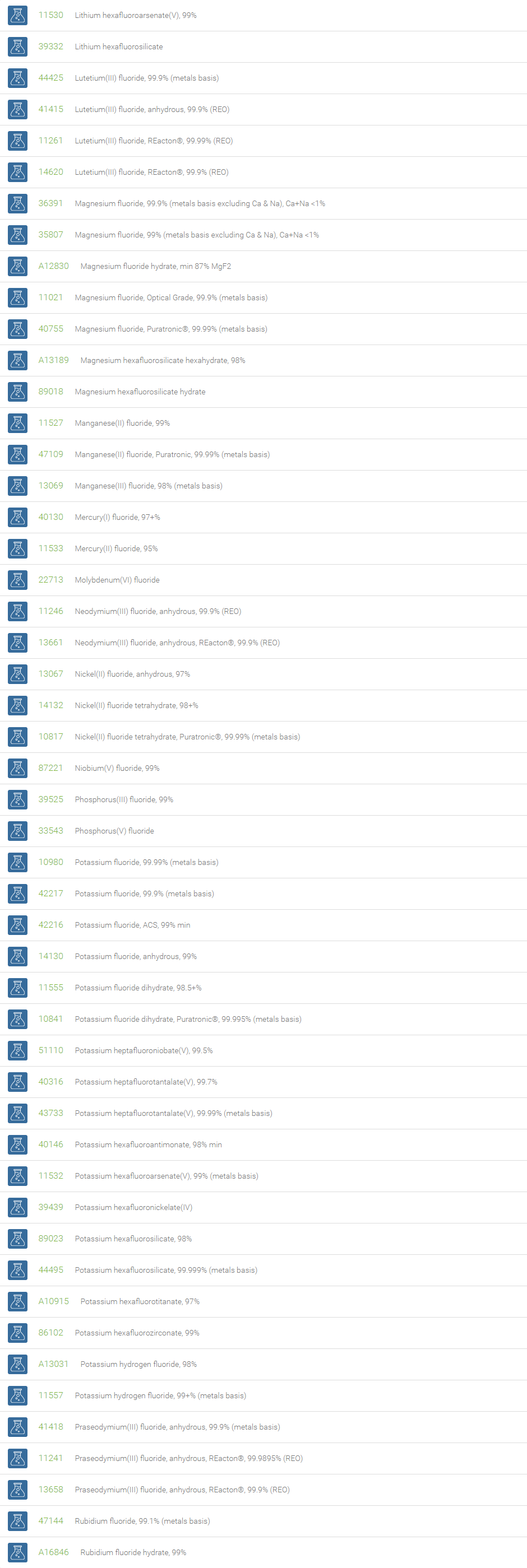
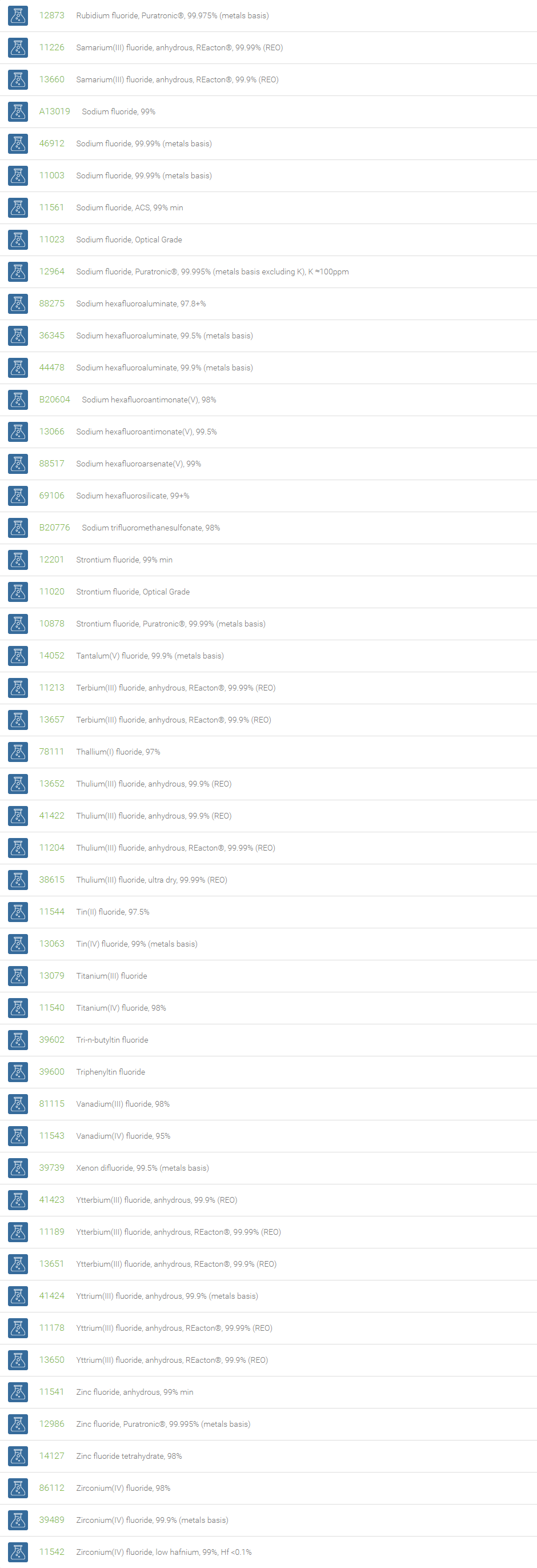
Inorganic Fluorides

Inorganic Fluorides
The inorganic anion of fluorine (F-) is called fluoride. The fluoride ion can form various inorganic compounds such as calcium fluoride, sodium fluoride, aluminum fluoride, potassium fluoride and magnesium fluoride. Bifluorides are also known to form with metals. Fluoride ions occur on earth in several minerals, particularly fluorite. Sodium fluoride was the first chemical used for fluoridation. Salts of fluoride are widely used as important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is the most bioavailable form of fluorine. Fluoride is necessary micronutrient for human health to prevent dental cavities and widely used as promoter for bone growth.
Fluoride is used in the manufacture of cryolite, Na3AlF6, which is required for aluminium smelting. Topical and systemic fluoride therapy is used for preventing tooth decay. Fluoride salts are commonly used in biological assay processing to inhibit the activity of serine/threonine phosphatases. Beryllium fluoride and aluminium fluoride are also used as phosphatase inhibitors. Bifluoride salts (HF2-) are used to etch glass surface. Some examples of bifluoride salts are ammonium bifluoride and potassium bifluoride. In analytical chemistry, the lanthanum fluoride electrode, which is an ion selective electrode containing lanthanum fluoride and europium fluoride, is very sensitive to the concentration of fluoride ions.