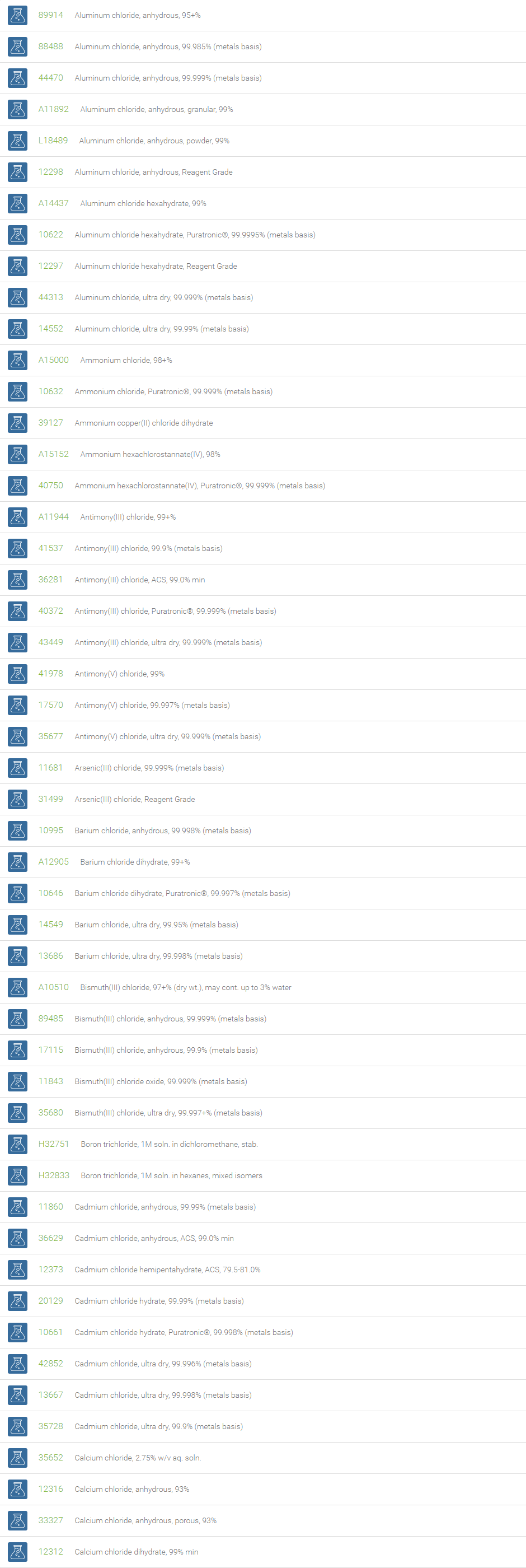
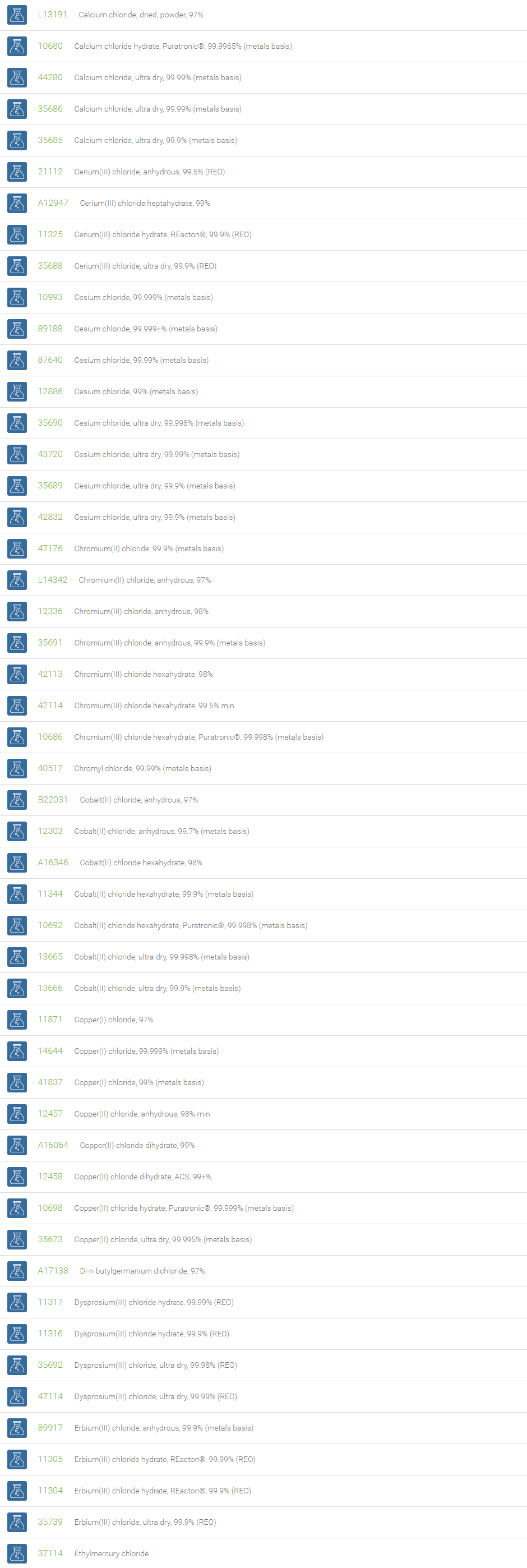
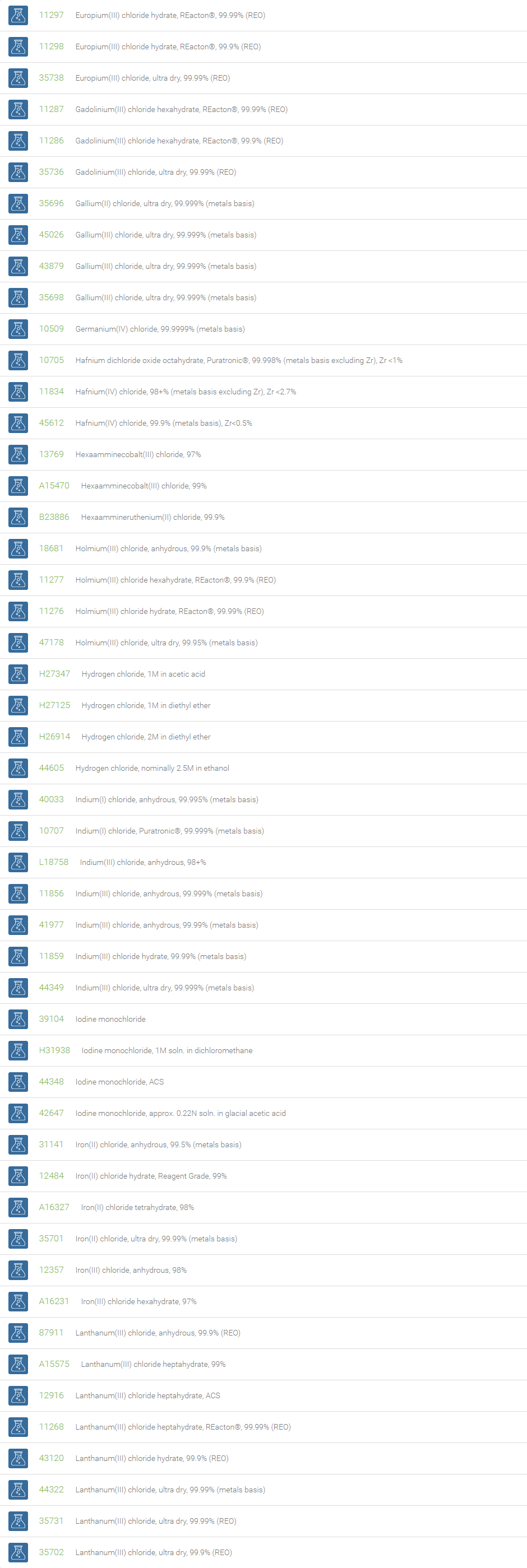
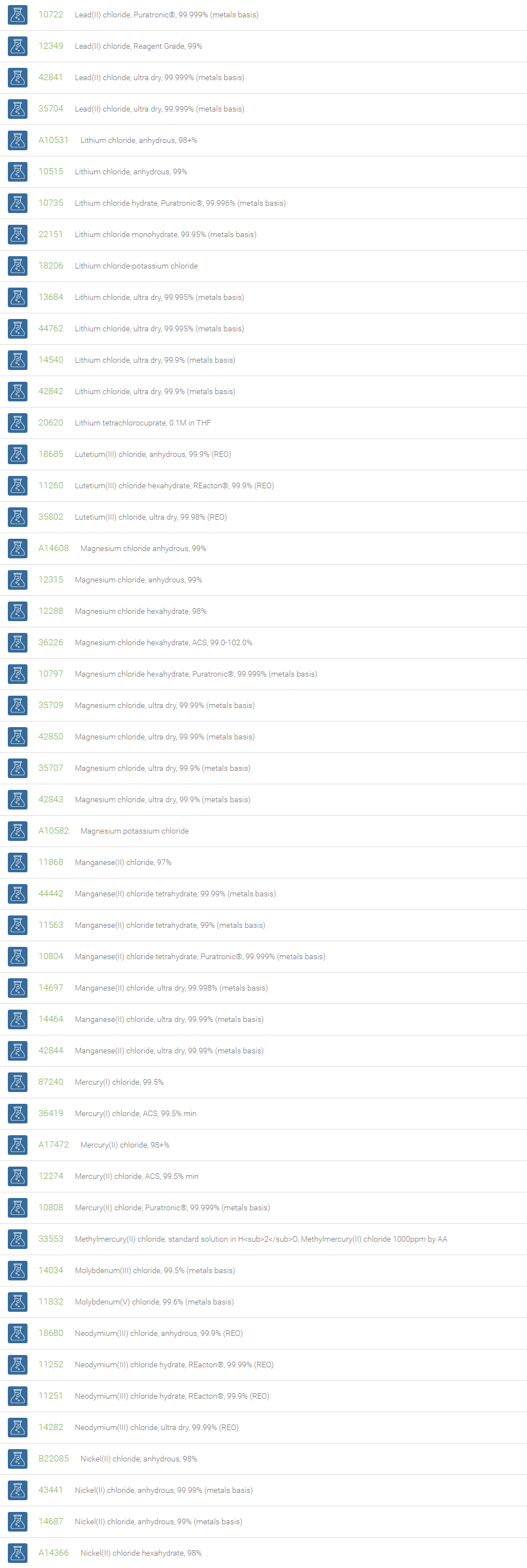
Inorganic Chlorides

Inorganic Chlorides
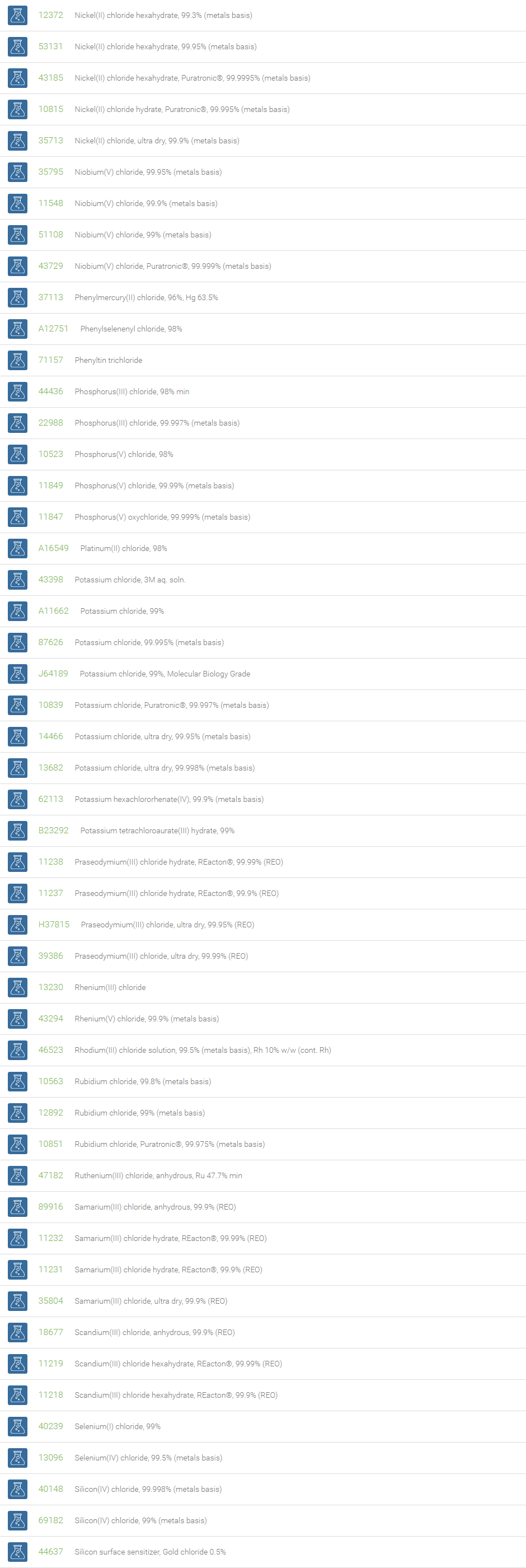
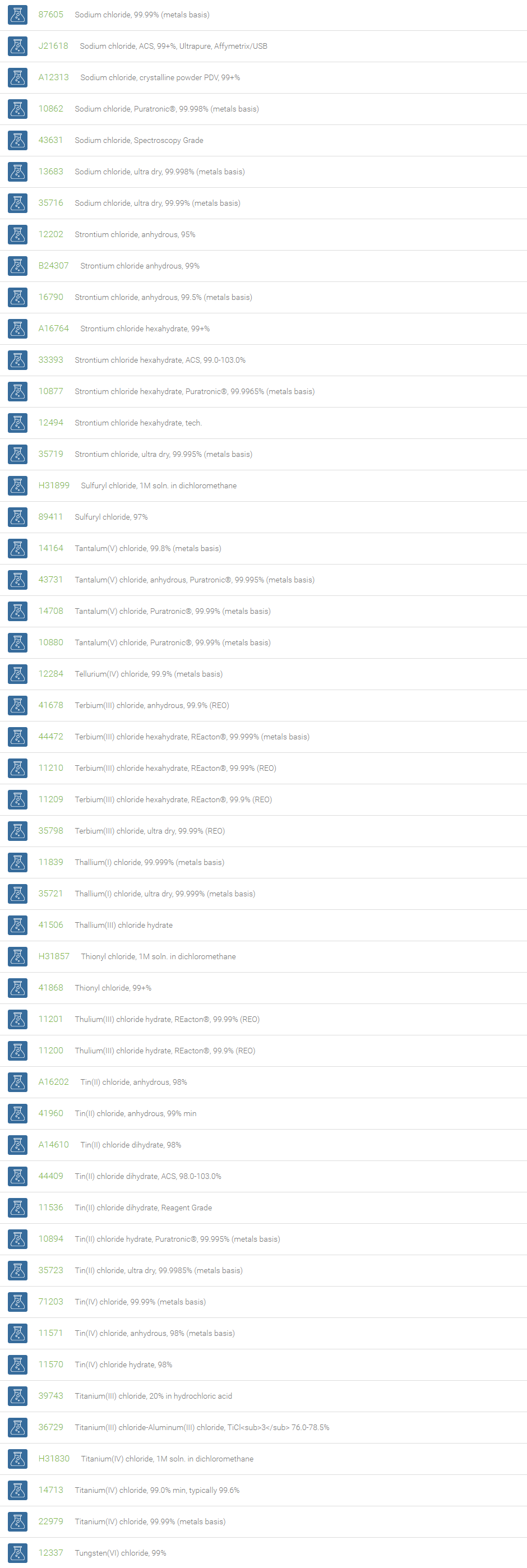
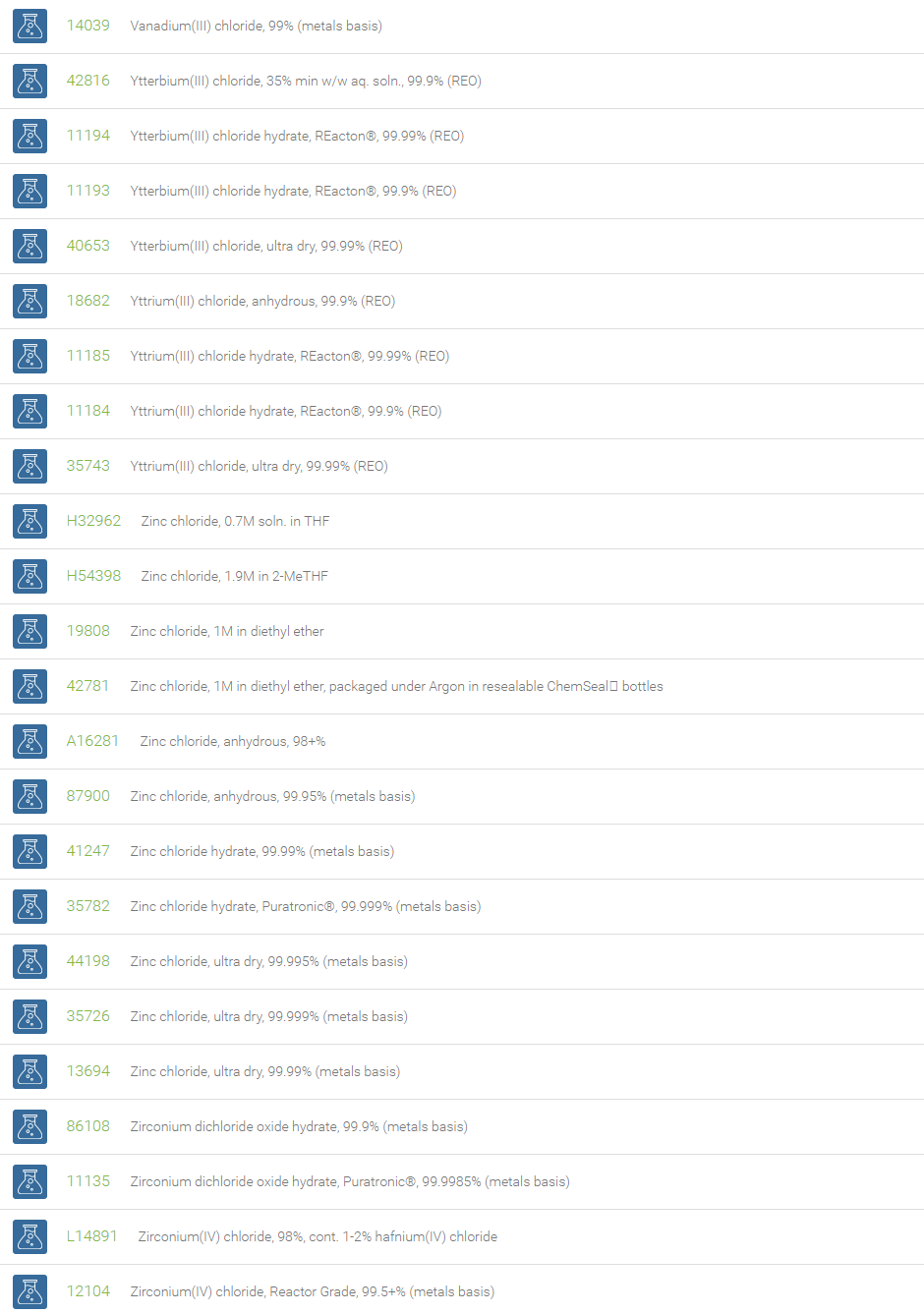
Chlorine forms compounds with the other halogens and with oxygen; when chlorine is the more electronegative element in the compound, the compound is called a chloride. The salts of hydrochloric acid contain chloride ions and can also be called chlorides. The word chloride can also refer to a chemical compound in which one or more chlorine atoms are covalently bonded in the molecule. The simplest example of an inorganic covalently bonded chloride is hydrogen chloride, HCl. Chloride is also a useful and reliable chemical indicator of river / groundwater fecal contamination, as chloride is a non-reactive solute and ubiquitous to sewage & potable water. A chloride ion is also the prosthetic group present in the Amylase molecule.
A chloride test measures the level of chloride in human blood or urine. Chloride is one of the most important electrolytes in the blood. It helps keep the amount of fluid inside and outside of your cells in balance. It also helps maintain proper blood volume, blood pressure, and pH of human body fluids. Most chlorides are salts that are formed either by direct union of chlorine with a metal or by reaction of hydrochloric acid with a metal, a metal oxide, or an inorganic base. Chloride salts include sodium chloride, potassium chloride, calcium chloride and ammonium chloride. Most chloride salts are readily soluble in water, but mercurous chloride (calomel) and silver chloride are insoluble, and lead chloride is only slightly soluble. Some chlorides such as antimony chloride and bismuth chloride decompose in water, forming oxychlorides. Many metal chlorides can be melted without decomposition; two exceptions are the chlorides of gold and platinum. Most metal chlorides conduct electricity when fused or dissolved in water and can be decomposed by electrolysis to chlorine gas and the metal.