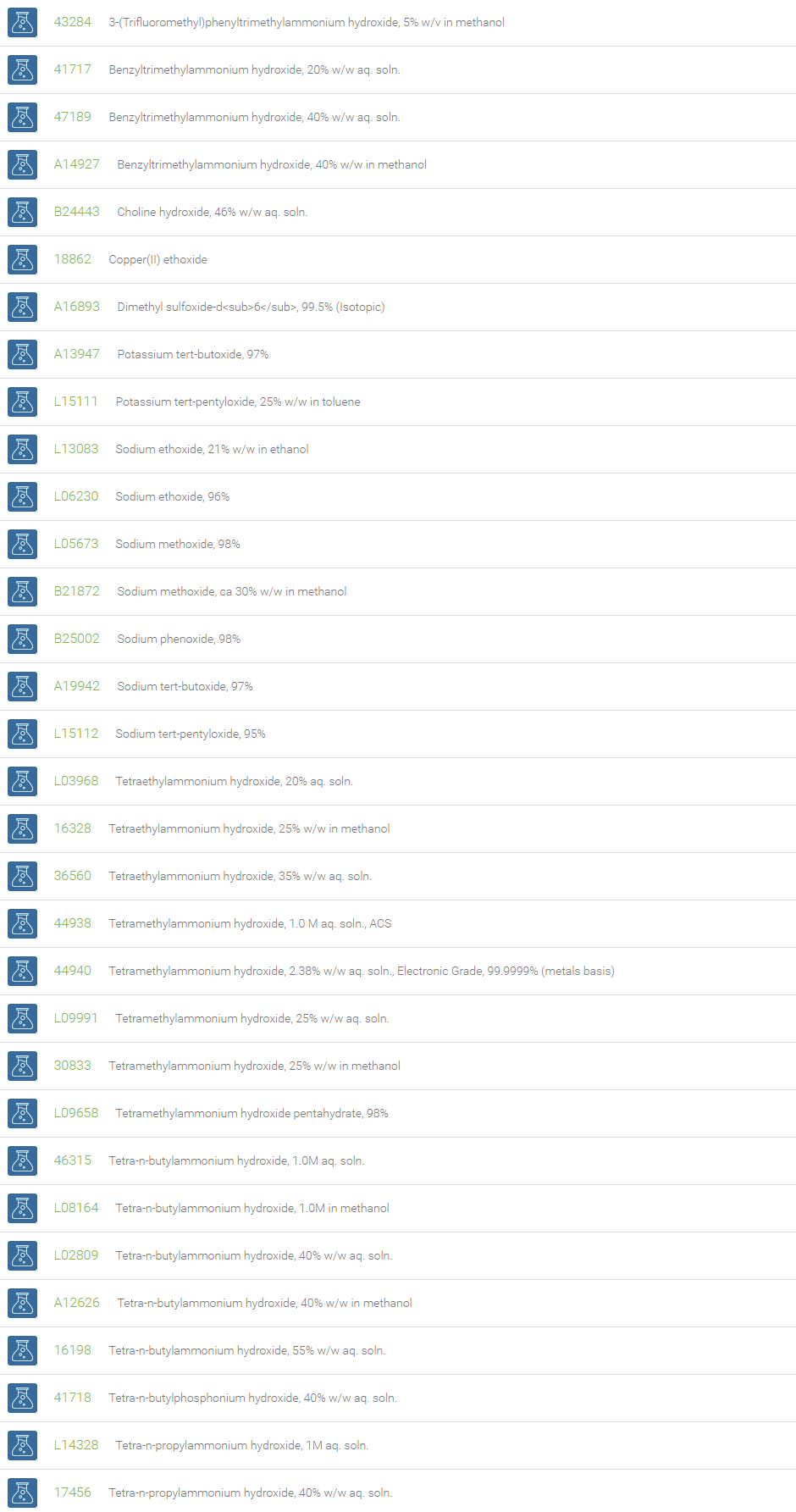
Organic Hydroxides

Organic Hydroxides
Organic hydroxides include quaternary ammonium hydroxides (R4N+ OH-), quaternary phosphonium hydroxides (R4P+ OH-) and tertiary sulfonium hydroxides (R3S+ OH-). These organic hydroxides may be collectively referred to as onium hydroxides. In both quaternary ammonium hydroxides and quaternary phosphonium hydroxides, there are four alkyl/aryl groups attached to nitrogen or phosphorus atoms. Some of the examples are tetrabutyl-ammonium hydroxide, benzyltriethylammonium hydroxide and n-butyltriphenylammonium hydroxide. In the case of sulfonium hydroxides, there are three groups attached to the sulfur atom.
Organic hydroxide compounds, owing to their strong basic nature, are used in phase transfer catalysis of alkylations and deprotonations. Tetrabutylammonium hydroxide, Bu4NOH, reacts with hydrofluoric acid to produce tetrabutylammonium fluoride, Bu4NF, which is a useful reagent for desilylation in organic solvents. Organic hydroxides have found a variety of uses including use as a titrant for acids in organic solvents and as a supporting electrolyte in polarography. They have been used extensively as a developer for photoresists in printed circuit board and microelectronic chip fabrication. They also find use in electronics. Many organic hydroxides find use as precipitation agents.