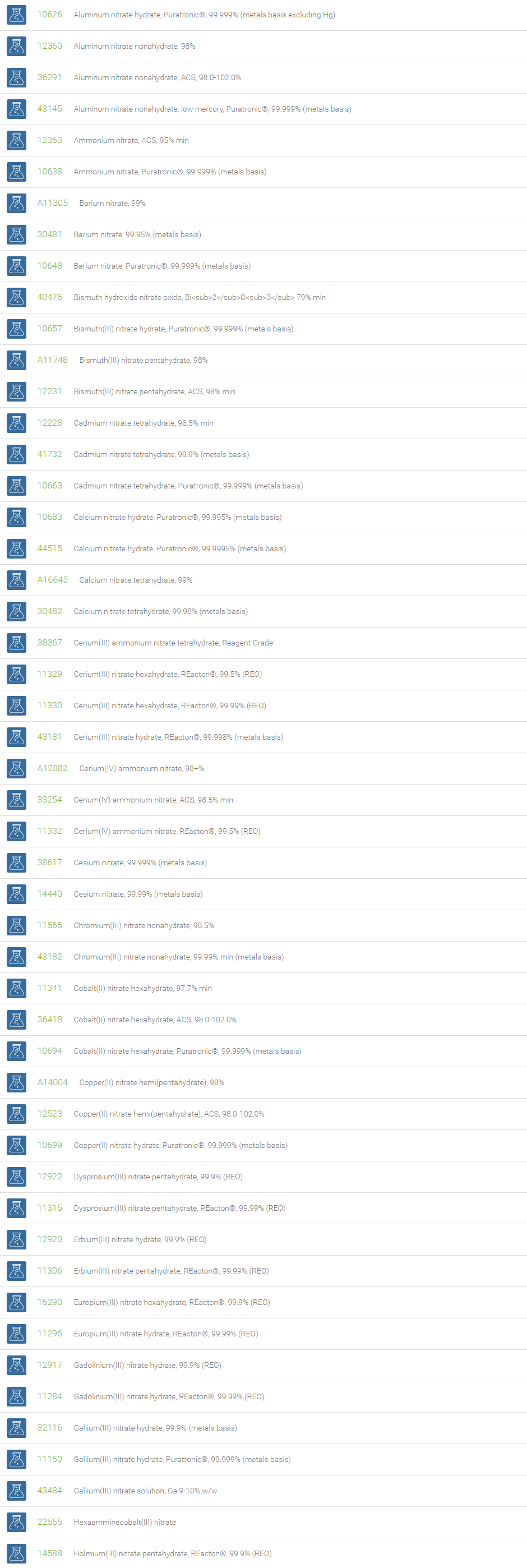
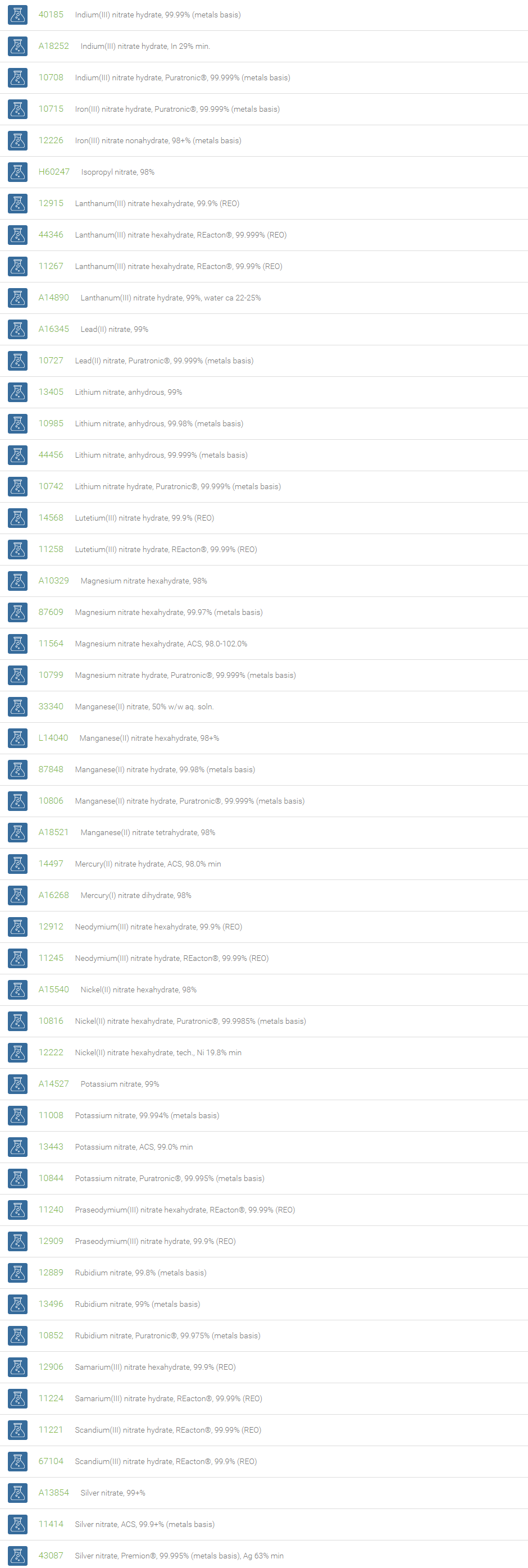
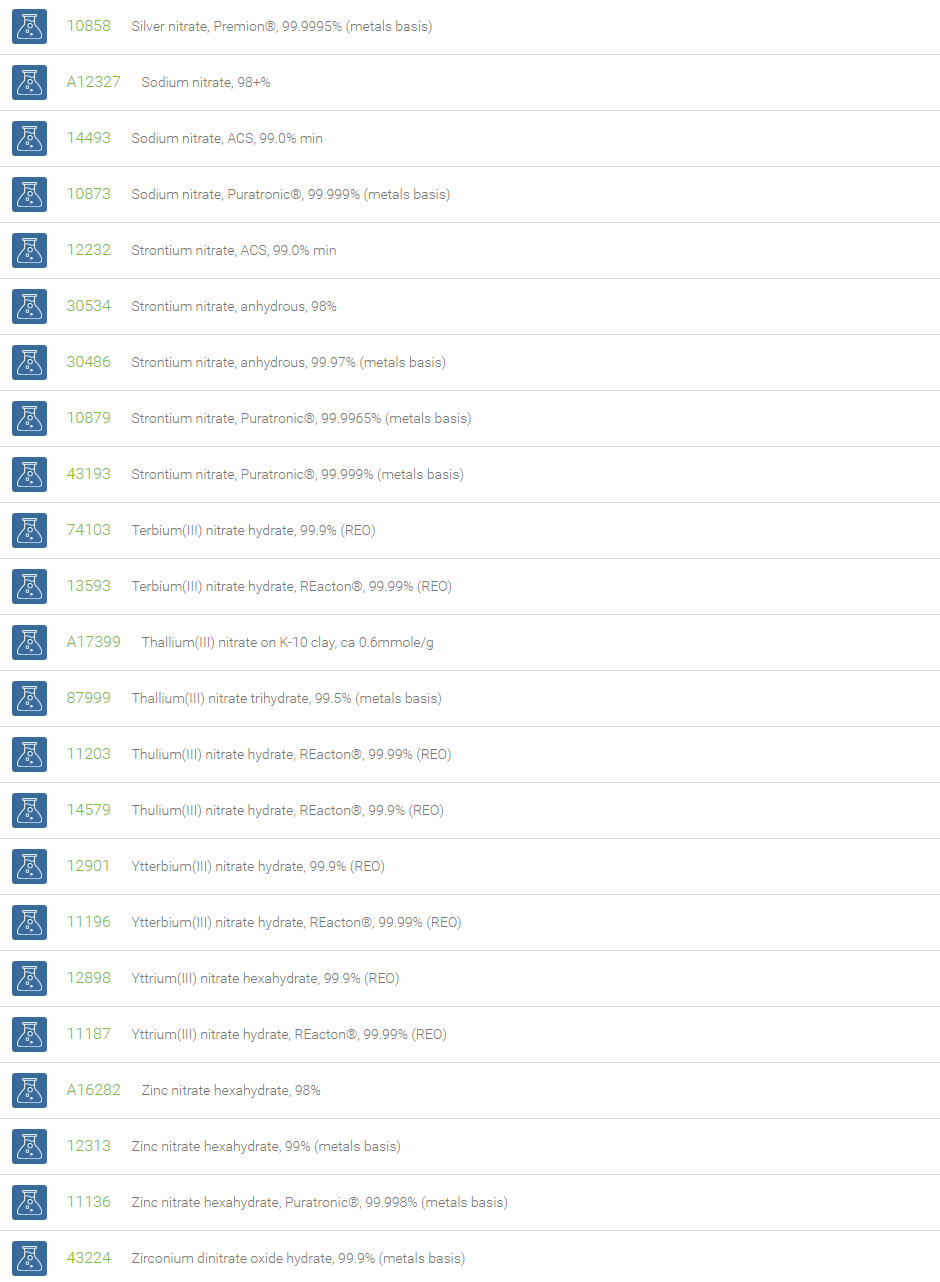
Nitrate Salts

Nitrate Salts
Salts of nitric acid containing the group NO3- are called nitrate salts. Nitrate anions form salts with a wide range of elements in the periodic table. Examples of nitrates are ammonium nitrate (NH4NO3), sodium nitrate (NaNO3) and potassium nitrate (KNO3). Nitrates consist of one central nitrogen atom surrounded by three identical oxygen atoms in a trigonal planar arrangement.
Nitrates are essential plant nutrients. The main use of nitrates is as fertilizers in agriculture. Nitrates are used as oxidizing agents. Sodium nitrate is used to remove air bubbles from molten glass in the manufacturing of high quality glass. Mixtures of the molten salt are used to harden some metals. Nitrates are used in certain specialty curing processes. Nitrates have been demonstrated to inhibit platelet aggregation and to mildly lower blood pressure. In organic chemistry, a combination of metal nitrate and sulfuric acid is a strong nitrating reagent, replacing fuming nitric acid mixture with sulfuric acid.