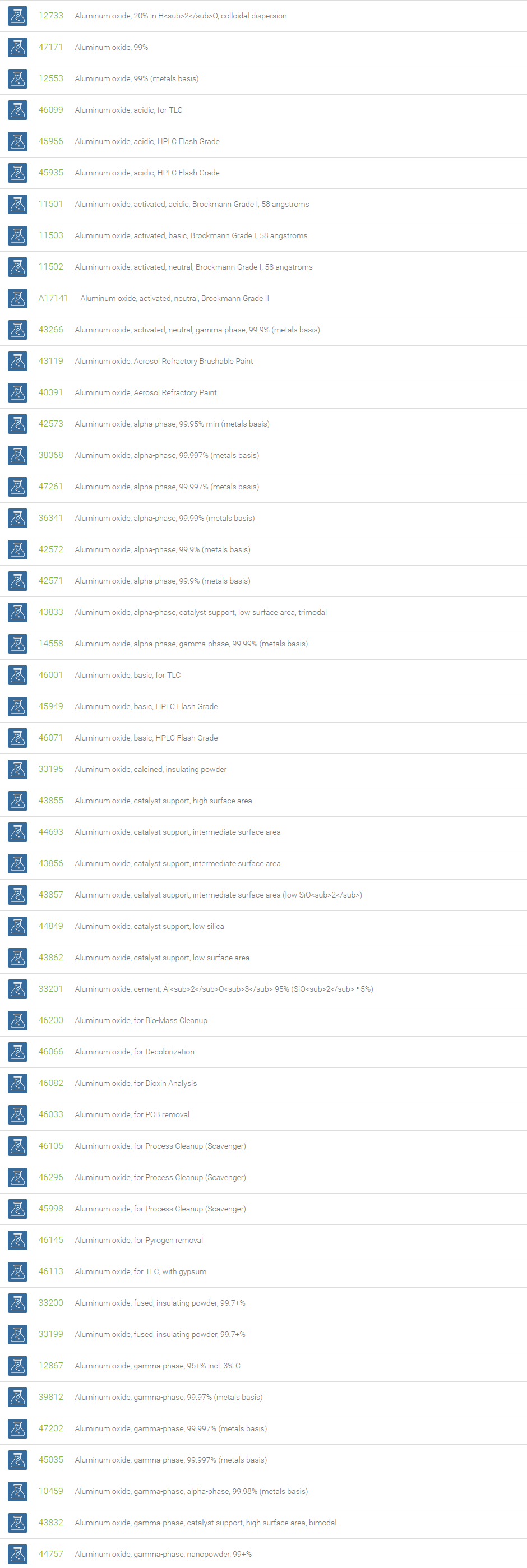
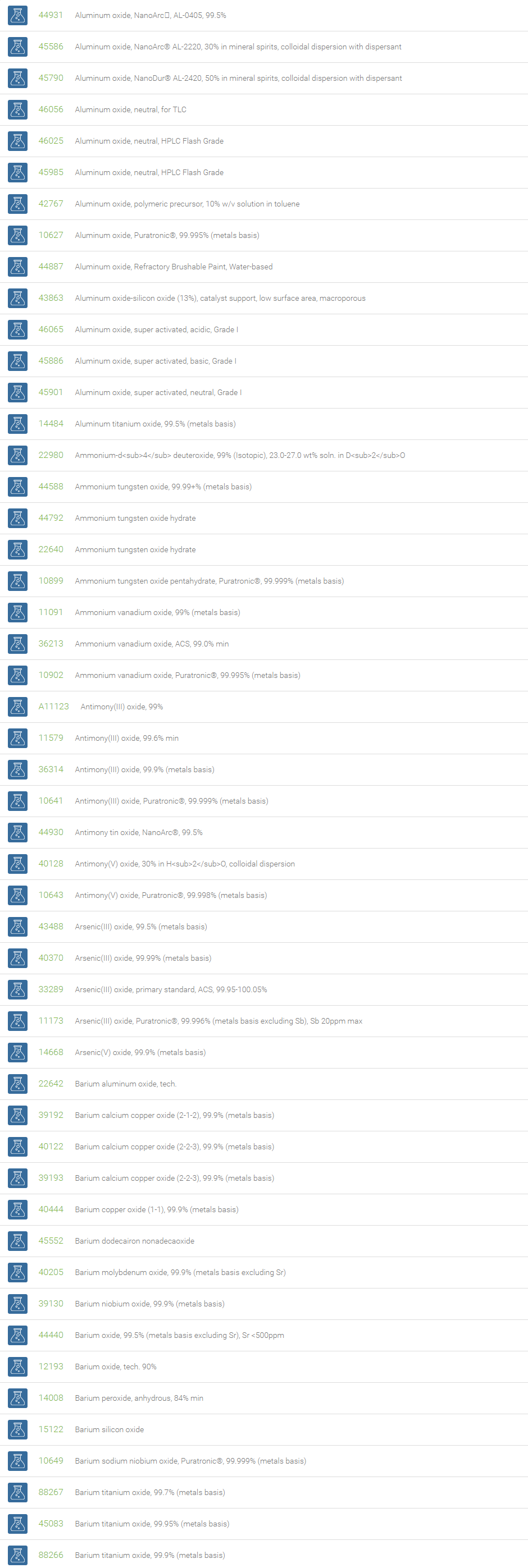
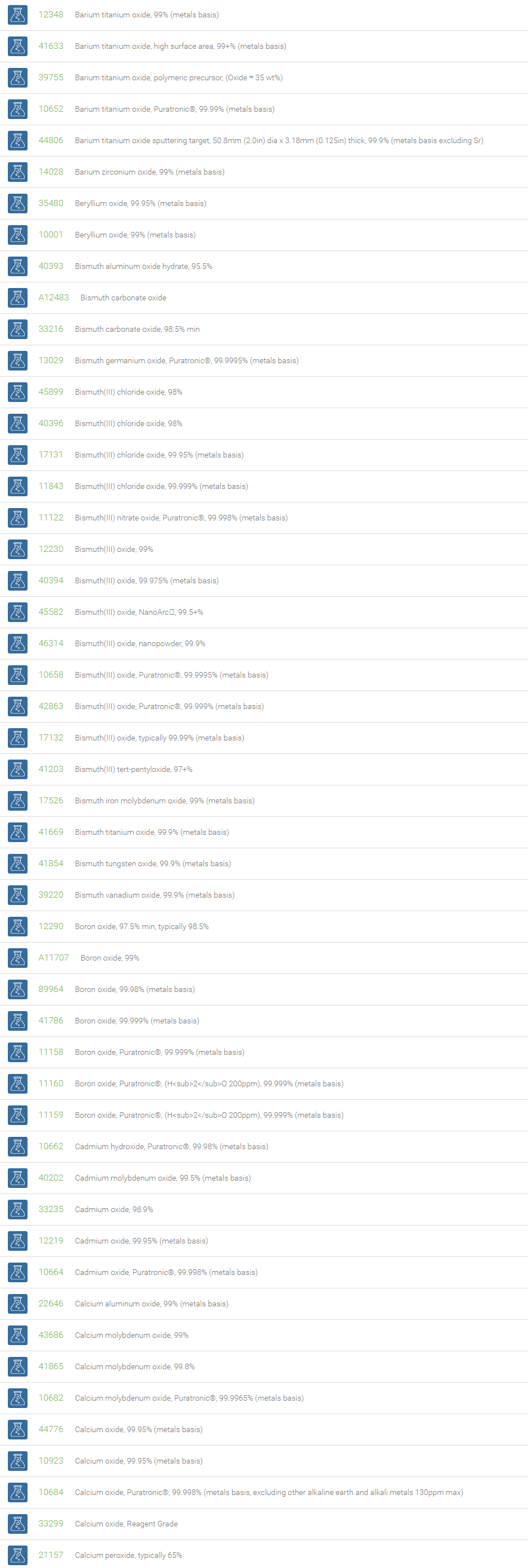
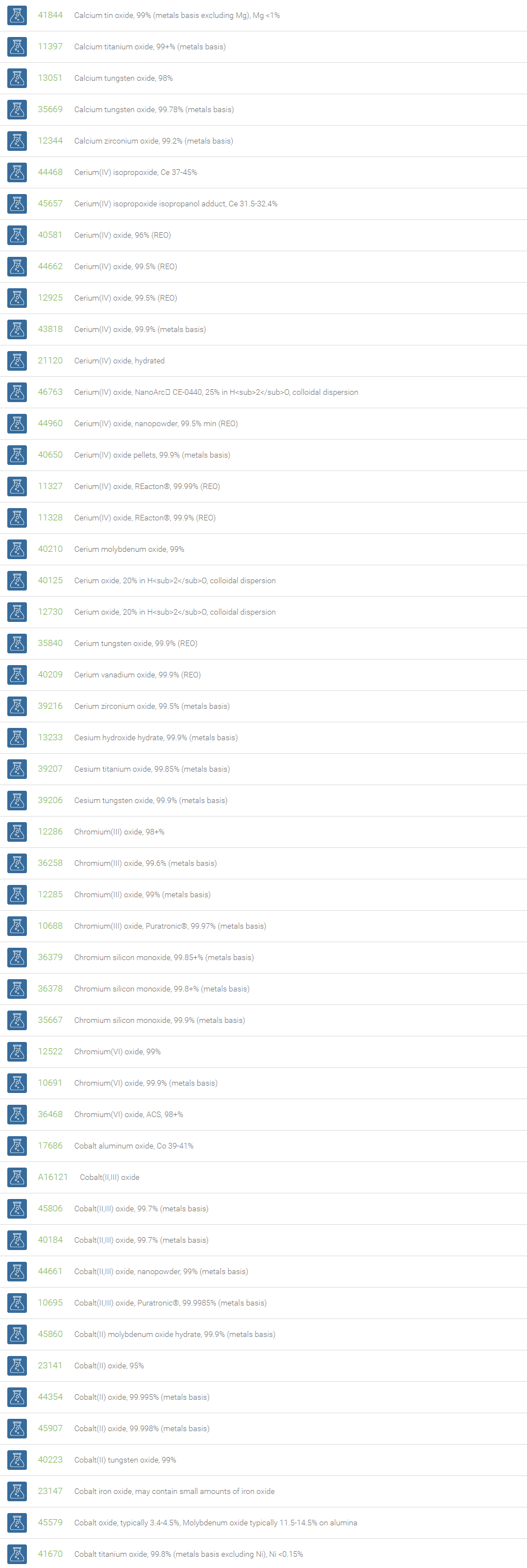
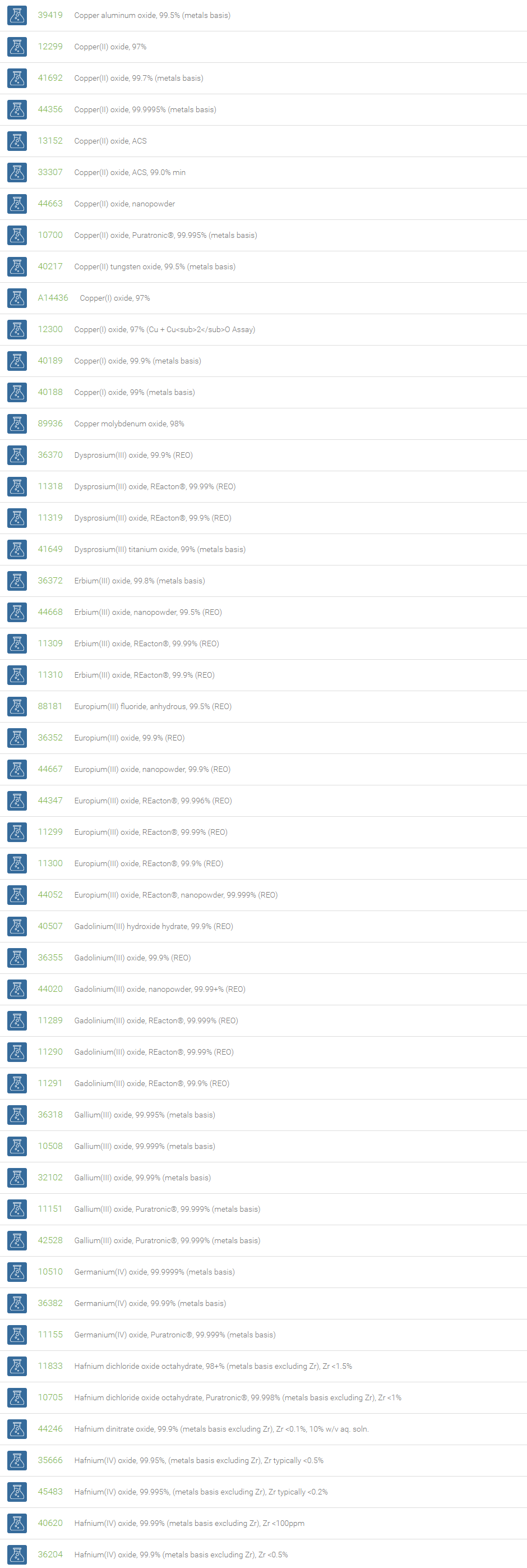
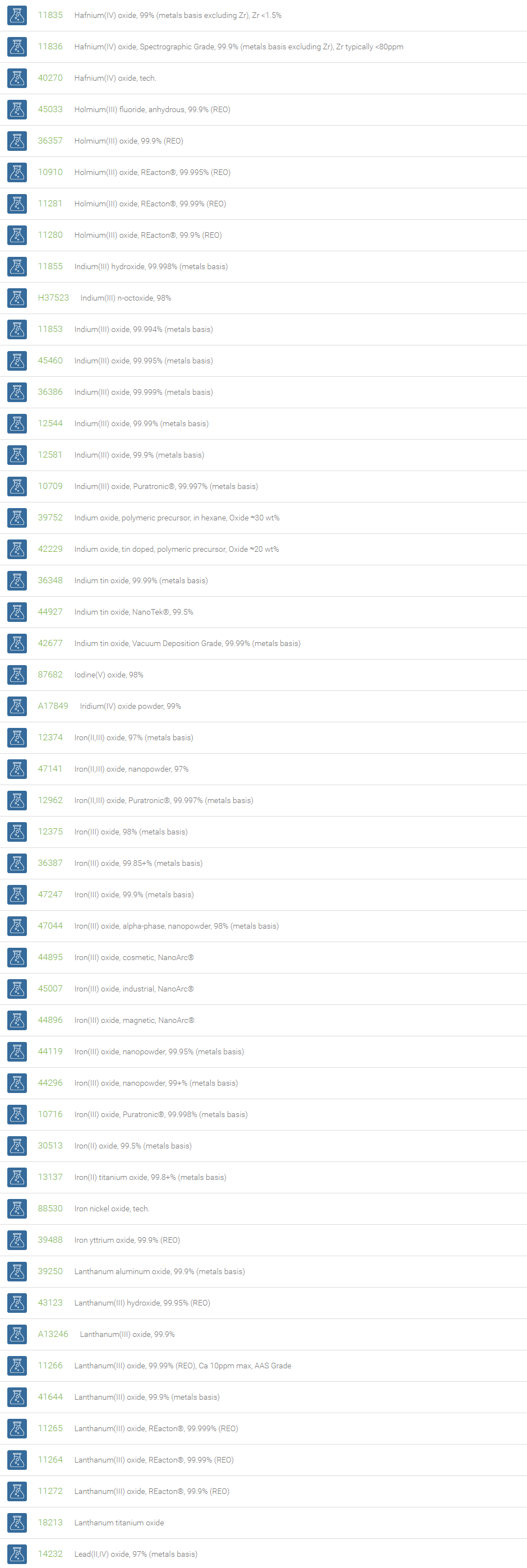
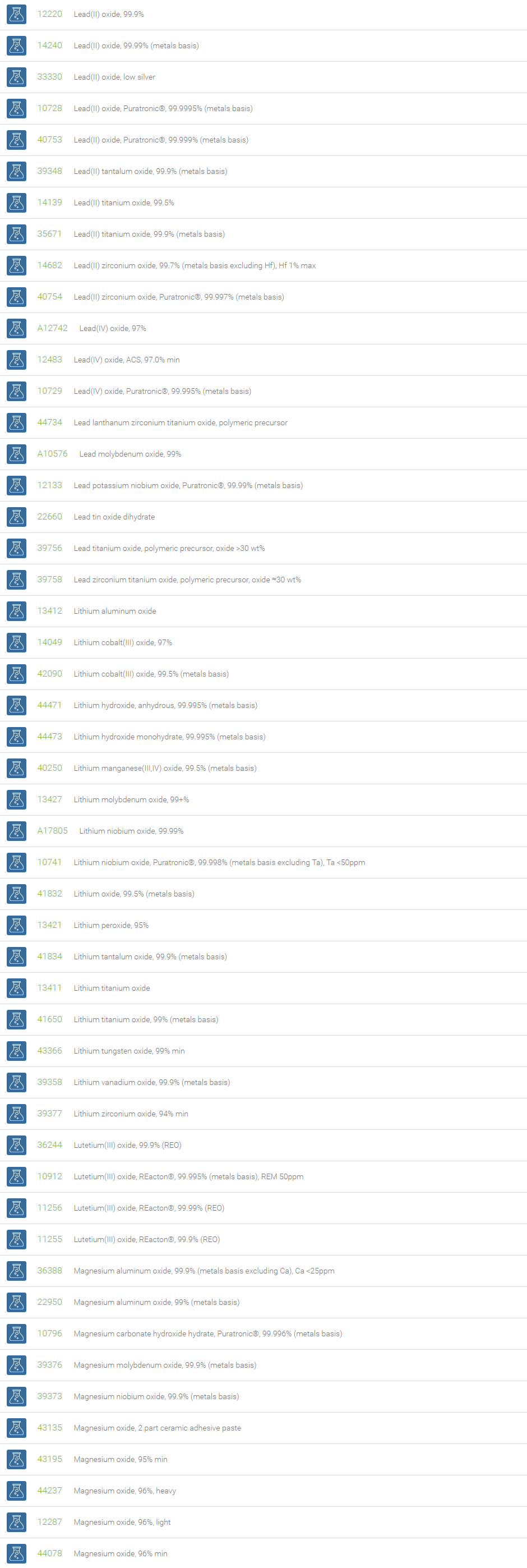
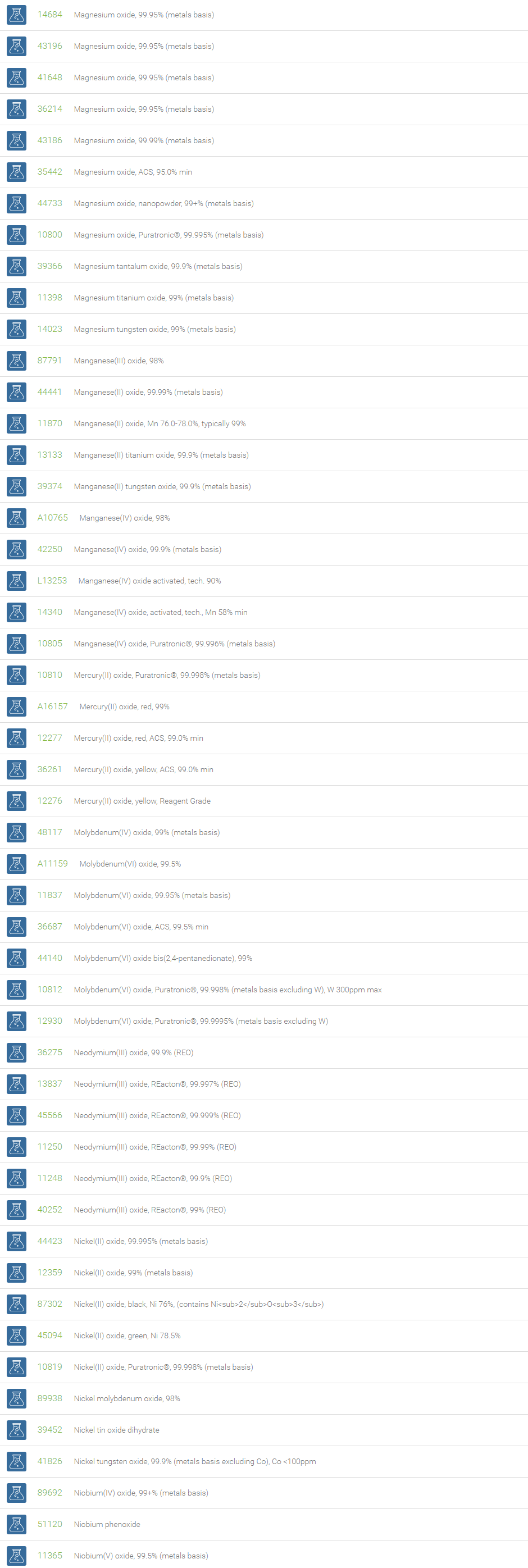
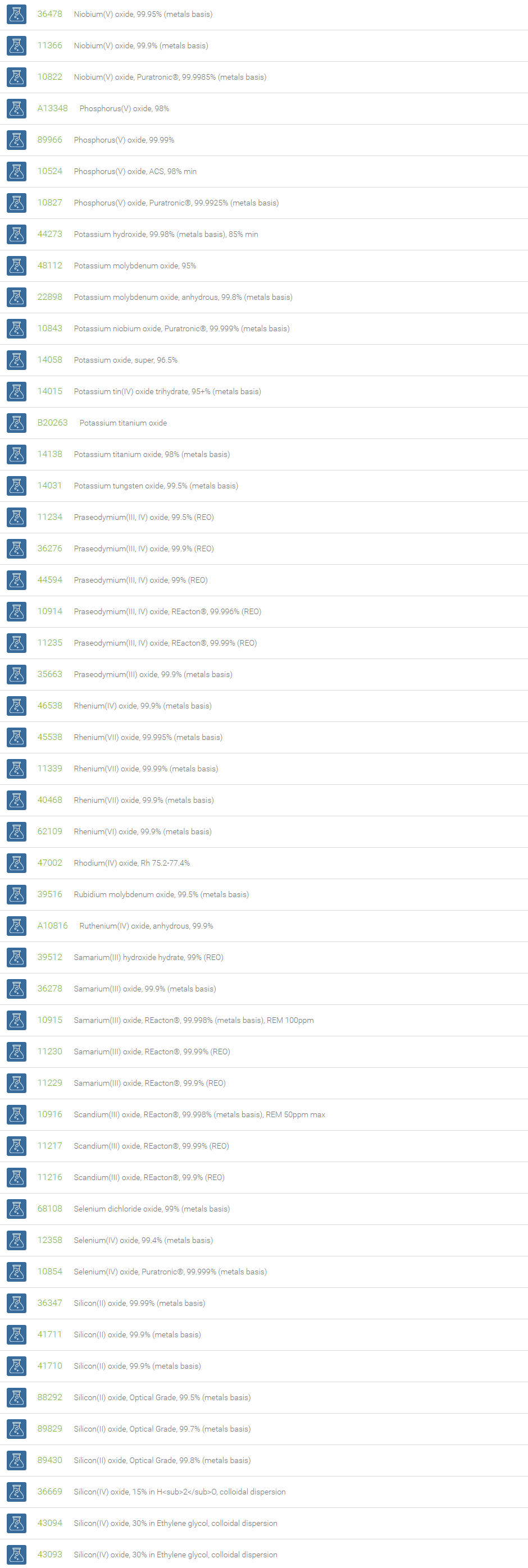
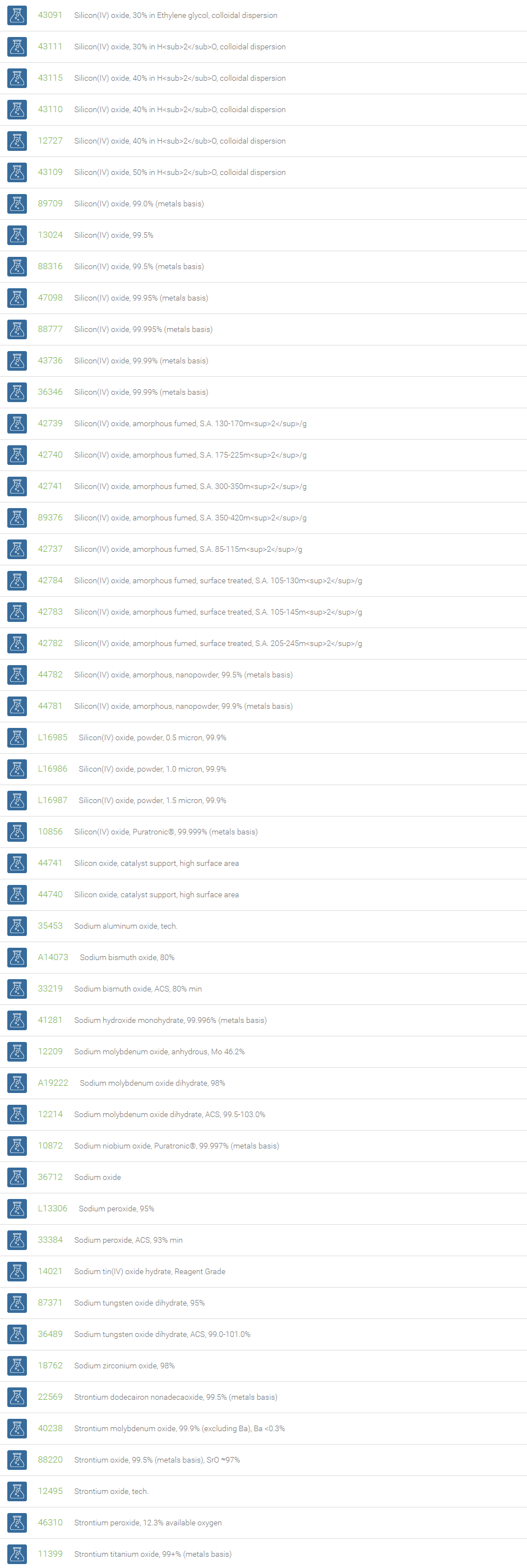
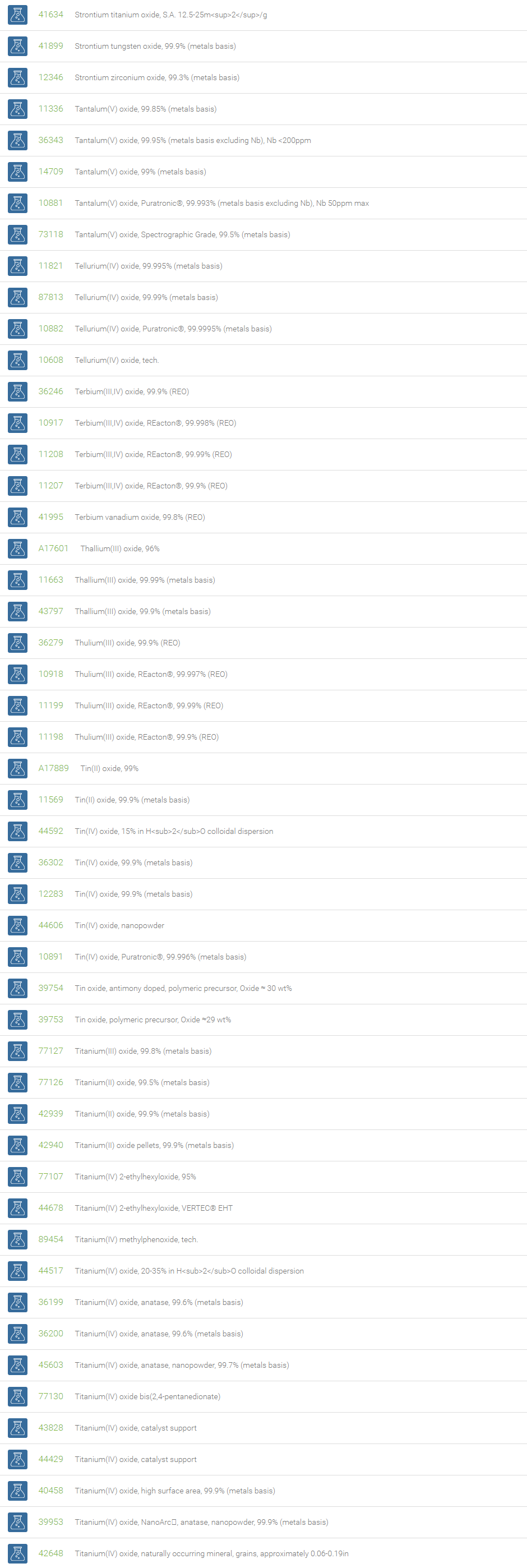
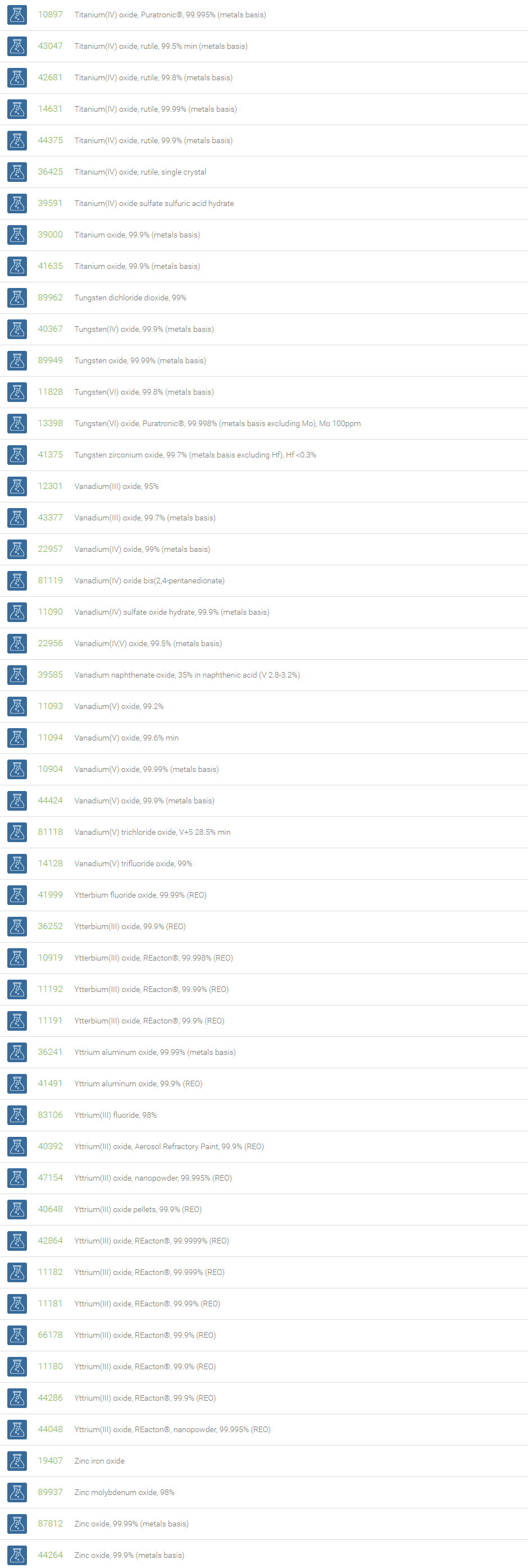
Inorganic Oxides

Inorganic Oxides
Inorganic oxides are binary compounds of oxygen with an element. Inorganic Oxides are classified into acidic or basic based on their acid-base characteristics.Acidic oxides gives an acid when combined with water. Examples of Acid oxides are Sulfurous acid, Carbonic acid and Sulfuric acid. Acid oxides are also known as acid anhydrides. Basic oxides gives a base in water. Generally Group I and II elements form basic oxides. Examples of Basic oxides are Copper (II) oxide, Magnesium oxide and Aluminum oxide.
Amphoteric oxides are metallic oxides which show both basic as well as acidic properties. When they react with an acid, they produce salt and water, showing basic properties. While reacting with alkalies they form salt and water showing acidic properties. A common example of an amphoteric oxide is aluminum oxide. Neutral oxides which show neither basic nor acidic properties, They do not form salts when reacted with acids or bases. Carbon monoxide (CO); nitrous oxide (N2O); nitric oxide (NO) are neutral oxides. Preparation: Inorganic Oxides can be prepared by direct hearing of element with oxygen, reaction of oxygen with compounds at high temperatures, oxidation of metals and non-metals with Nitric acid. In a periodic table, the oxides of elements in a period become progressively more acidic as one goes from left to right, Basic oxides are present on the left side and acidic oxides are found on the right side of the periodic table.