Inorganic Cyanides

Inorganic Cyanides
Inorganic cyanides are compounds which are salts of hydrocyanic acid. Inorganic cyanides have the cyano group bounded to metal atoms. They are all white solids, and soluble in water. In acidic water, inorganic cyanides liberate gaseous hydrogen cyanide. Inorganic cyanides are incompatible with isocyanates, nitrides and peroxides. Due to their high nucleophilicity, cyanide ions can be introduced into organic compounds by replacement of halides, or other leaving groups. Cyanide can be used to lengthen a carbon chain by one unit.
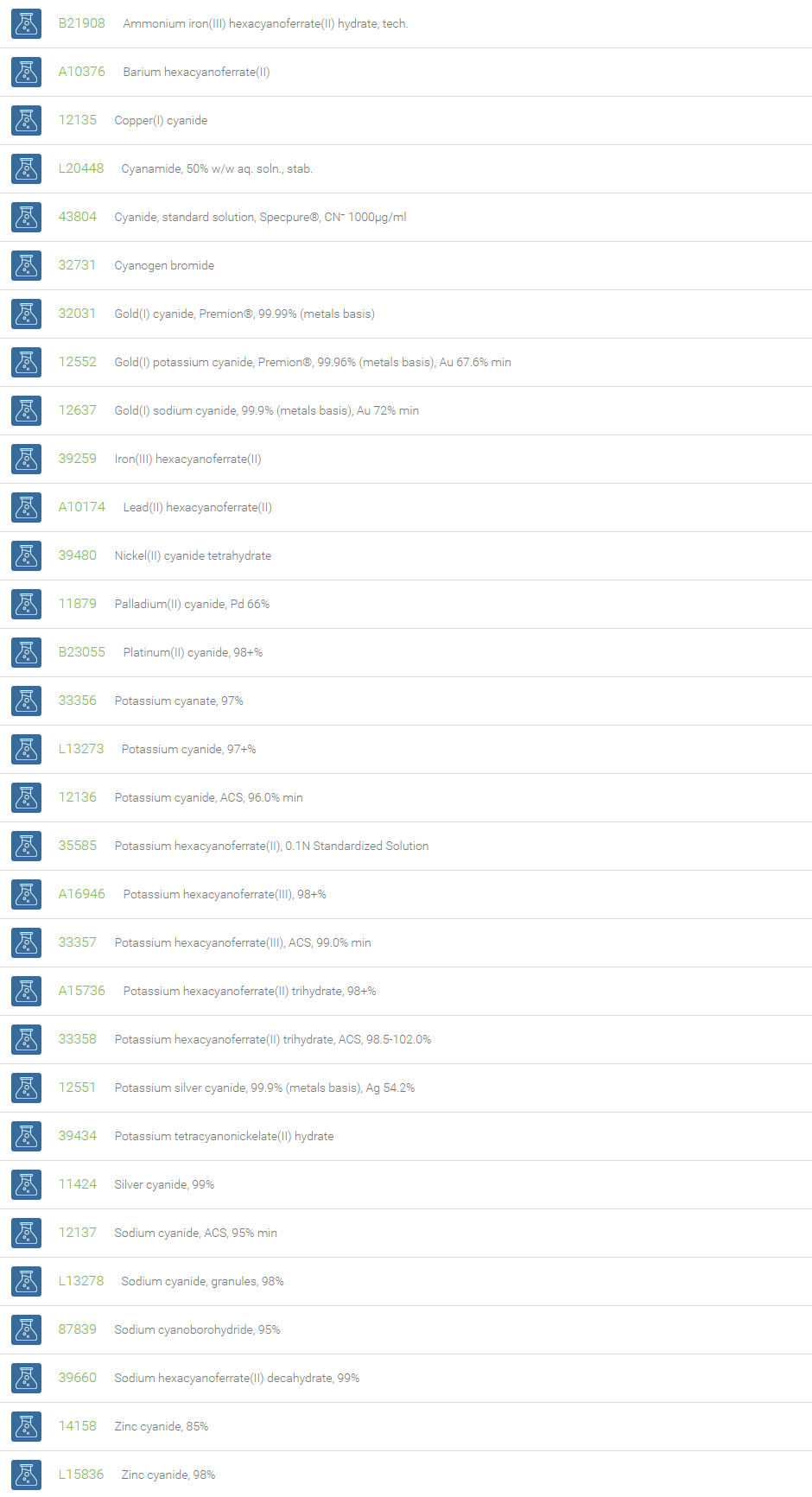
Examples of inorganic cyanides include: calcium cyanide, potassium cyanide, sodium cyanide, lead cyanide, potassium cyanide, and silver cyanide. Some commercial applications of inorganic cyanides are in electroplating, metallurgy, photographic processes, the extraction of ores (gold and silver), and tanning leather. In nature, substances yielding cyanide are present in certain seeds, such as the pit of the wild cherry and the seeds of apples. Cyanide salts find use in the manufacture of important reactive compounds, such as methyl methacrylate, adiponitrile and acrylonitrile, and hence are useful in the production of acrylic fibres, synthetic rubber, and plastics. Metals have a high affinity to the cyanide anion due to its compactness, negative charge, and ability to form pi-boning. Many coordination compounds contain cyanide as ligand, for example, Prussian blue, hexacyanides, tetracyanides and dicyanides.