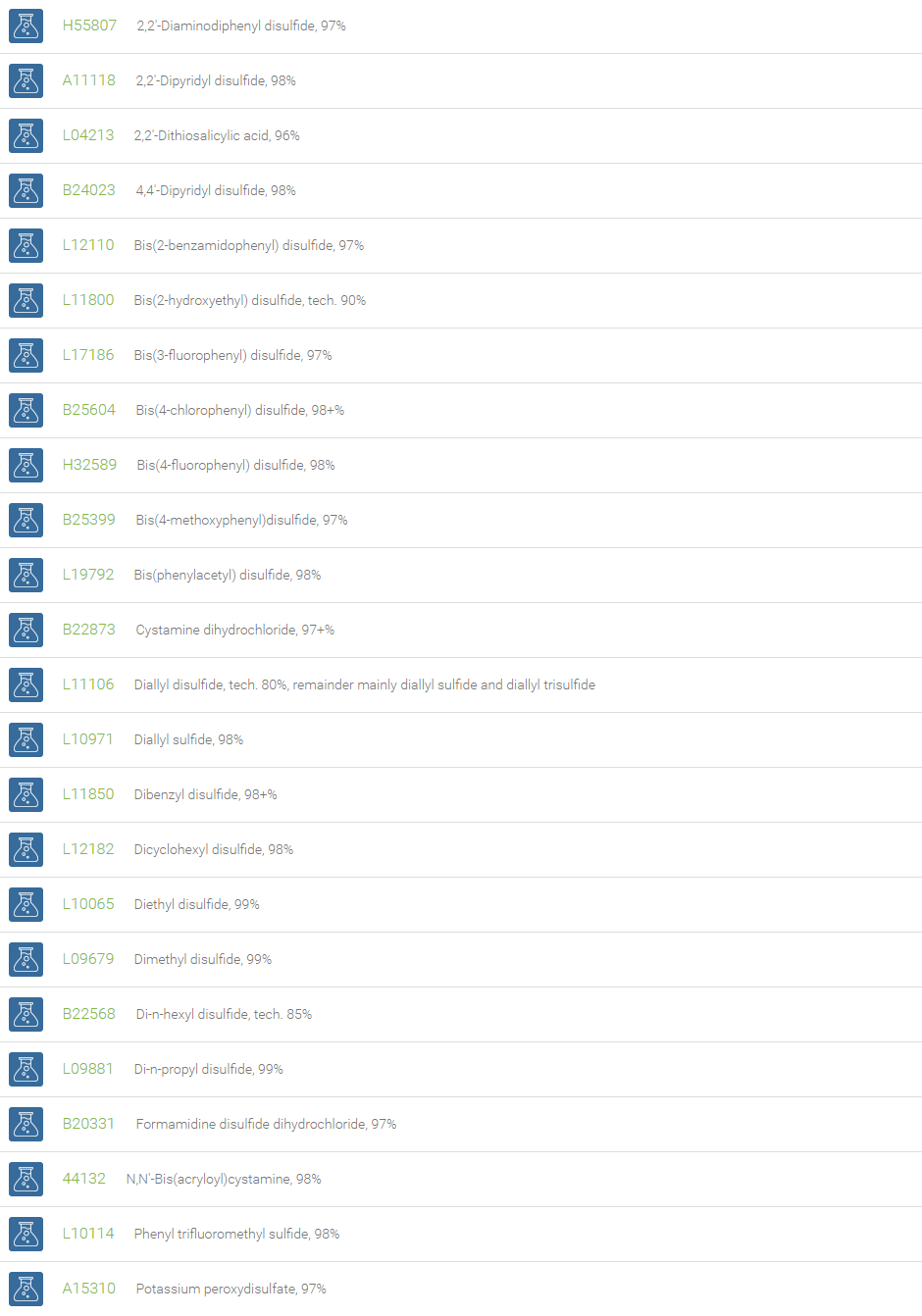
Disulfides

Disulfides
In organic chemistry, disulfide refers to that class of carbon compounds which contain a sulfur-sulfur linkage. Glutathione disulfide is a naturally occurring disulfide. Disulfide bonds are weaker than C-C and C-H; and are the weak link in an organic disulfide. They are attacked both by nucleophilic and electrophilic reagents. In organic synthesis, disulfides are used to prepare other sulfur containing compounds. Aryl disulfides can be used to activate carboxylic acid as aryl thioesters for the subsequent preparation of substituted amides, as often used in beta-lactam antibiotic chemistry. With chlorine under milder conditions, disulfide forms sulfenyl chlorides while under harder conditions they form sulfonic acid. Bis(2-aminophenyl) disulfides are a potential precursor to the synthesis of 2-aminobenzenethiols.
Dibenzyl disulfide finds utility in the manufacturing of fragrance compounds, high-pressure lubricant additives, and corrosion inhibitors. Alkyl disulfides are generally employed to prepare highly ordered monolayers. The property of such monolayers can be attuned by changing the chemical nature of the terminal groups. Such self-assembled monolayers of disulfides find use in modern micro and nano-fabrication, biomaterials, molecular electronics, and sensory applications. Disulfide linkages are frequently encountered in proteins and as cross-linkers during the vulcanization of rubber. Pyridine disulfides, as sulfhydryl cross-linkers, are employed to introduce disulfide linkages in proteins that already have a sulfhydryl group through a disulfide exchange reaction.