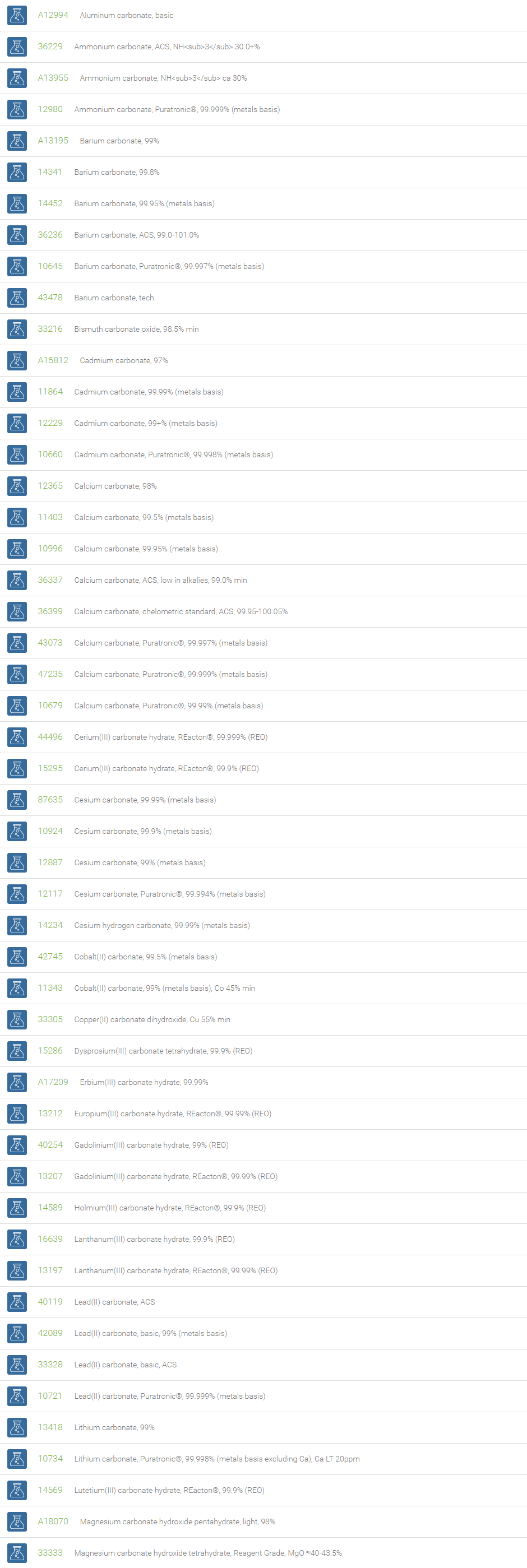
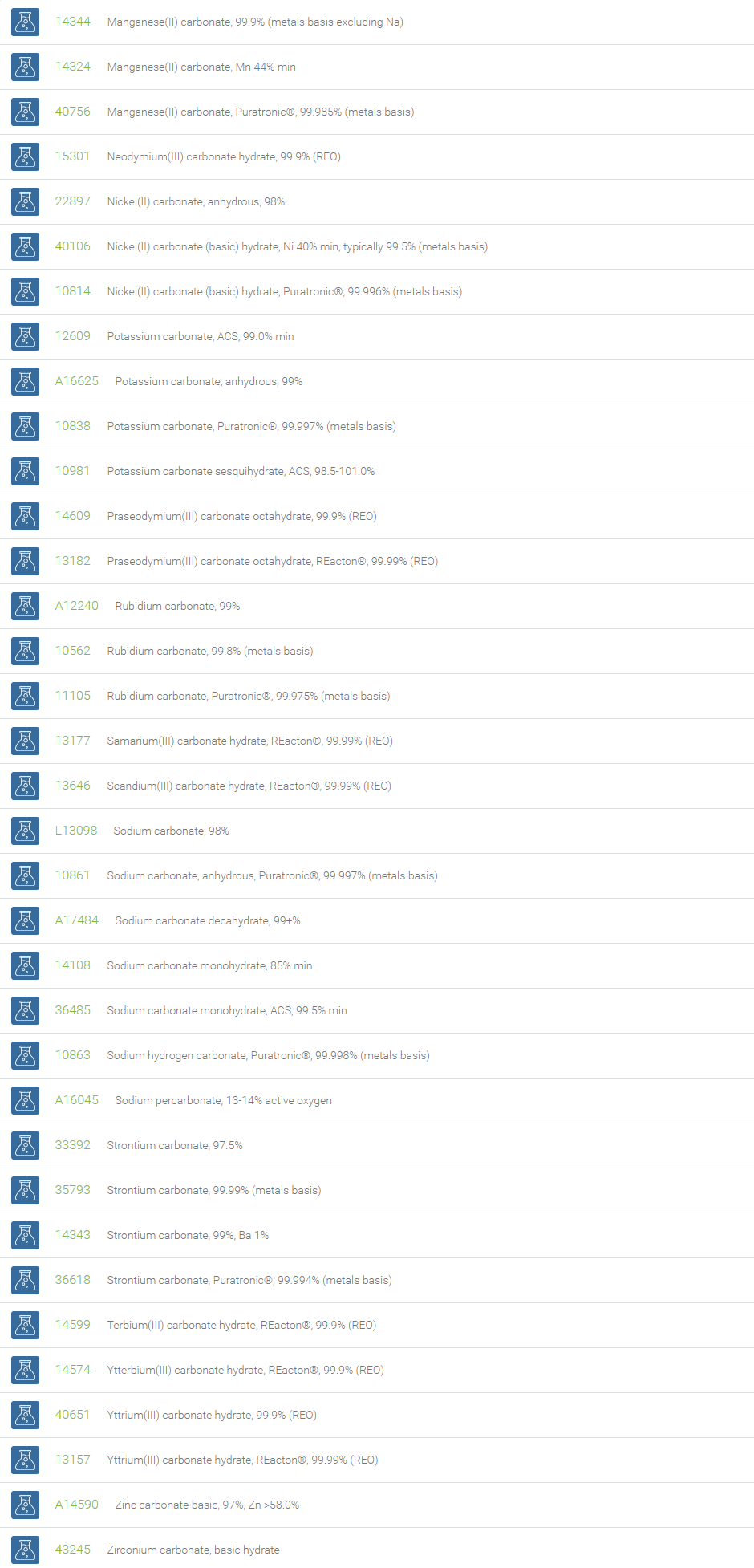
Carbonates

Carbonates
Inorganic carbonates are ionic compounds containing carbonate (CO32-) and metal ions. Some important carbonates are Li2CO3, Na2CO3, K2CO3, MgCO3, CaCO3, BaCO3, Tl2CO3, PbCO3, ZnCO3, CuCO3, Ag2CO3 and Fe2CO3. Group-1 and 2 elements form colorless carbonates and transition element carbonates may be colored. Most carbonate salts are insoluble except lithium, sodium, potassium, ammonium and uranium carbonates. On heating carbonates decompose to carbon dioxide and oxide.
Inorganic carbonates are used as raw materials in several industrial products/processes such as glass making, drug development, paper and pulp processing, silicates, soap and detergent production, water softener, drying agent, fireproofing, fire extinguishing compositions, clay and concrete production. Mmagnesium carbonate is used in antacid, table salt and tooth paste preparations. Lithium carbonate is used in batteries and essential medicines. Sodium percarbonate is used in some eco-friendly cleaning products and as a laboratory source of anhydrous hydrogen peroxide. Cesium carbonate is a base of choice for synthetic organic chemists for large number of N-alkylations of sensitive compounds; for clean oxidation of alcohols into carbonyl compounds; for Suzuki, Heck, and Sonogashira couplings.